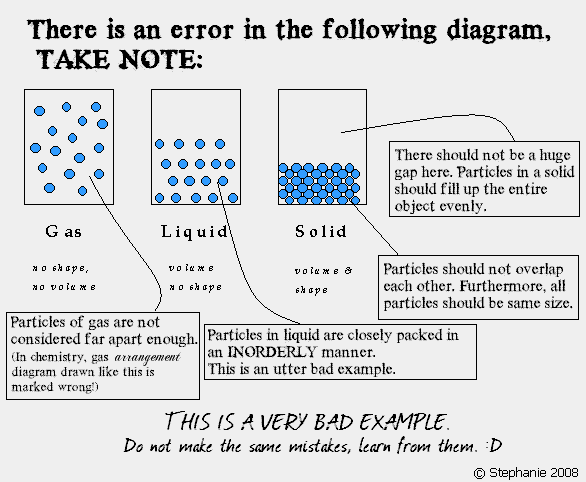
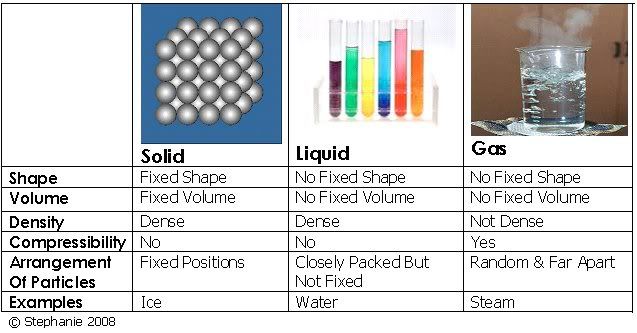
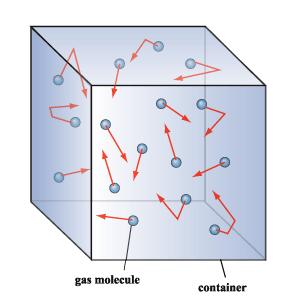
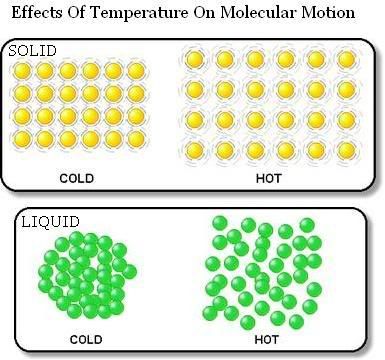
STEPHANIE loo. (30) 3B1. PLMGSS. Her usual page: ENTER? IN REGISTER NUMBER ORDER. 3B1 3B1 `08 AmandaLiew (01) LeeHuang (03) Chara (04) Cherlyn (05) Christina (06) XinYan (07) WenShi (08) Clarice (09) Jolene (10) Germaine (11) Clara (12) Isabel (13) Leah (15) AmandaLim ENMIAN (16) ZiAi (17) Maureen (18) Melissa (19) MiiWei (20) LiYing (22) NeoYun (23) WanLeng (24) Samantha (25) EnYin (26) Sheryl (28) Shobana (29) ♥ you are here-> STEPHANIE (30) Jesslyn (31) Valerie (32) YuXian (33) YingChing (34) QingHui (35) Cassandra (36) Vanessar (37) JiaYee (38) XiaoWei (39) KaiJing (40) Joaquim (41) 3B2 Eileen (09) ErnHuei (19) ElizabethTan (34) |
|