STEPHANIE
loo.
(30)
3B1.
PLMGSS.
Her usual page:
ENTER?
IN REGISTER NUMBER ORDER.
3B1
3B1 `08
AmandaLiew (01)
LeeHuang (03)
Chara (04)
Cherlyn (05)
Christina (06)
XinYan (07)
WenShi (08)
Clarice (09)
Jolene (10)
Germaine (11)
Clara (12)
Isabel (13)
Leah (15)
AmandaLim ENMIAN (16)
ZiAi (17)
Maureen (18)
Melissa (19)
MiiWei (20)
LiYing (22)
NeoYun (23)
WanLeng (24)
Samantha (25)
EnYin (26)
Sheryl (28)
Shobana (29)
♥ you are here-> STEPHANIE (30)
Jesslyn (31)
Valerie (32)
YuXian (33)
YingChing (34)
QingHui (35)
Cassandra (36)
Vanessar (37)
JiaYee (38)
XiaoWei (39)
KaiJing (40)
Joaquim (41)
3B2
Eileen (09)
ErnHuei (19)
ElizabethTan (34)
|
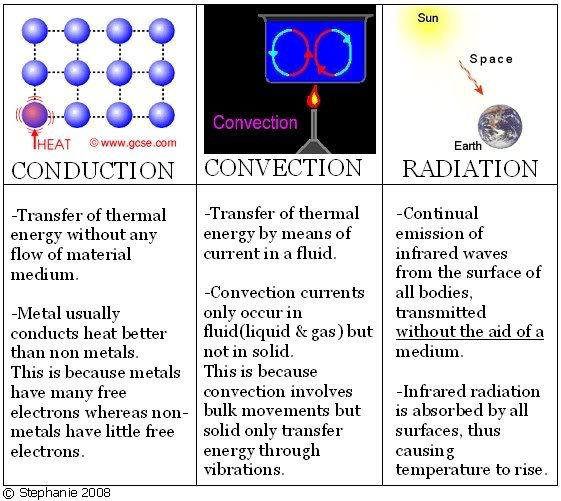
Unit 9 - Thermal Properties Of Matter
Objectives:
- Understand boiling, evaporation, condensation, melting and solidification.
- Understand what internal energy is.
- Applications of the different processes.
Internal Energy
Potential energy+Kinetic energy=Internal Energy!

Internal energy of a body is the energy stored in the body.
It is the sum of kinetic energy and potential energy of all particles in the body.
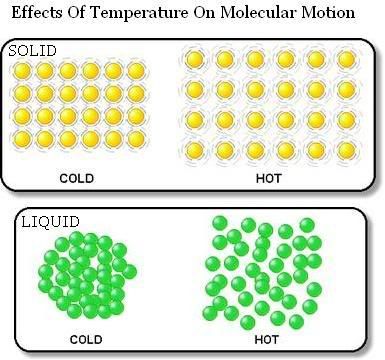
It increases with the temperature of the body and increases when the body changes state from solid to liquid and from liquid to gas.

The stretchable slinky have internal energy stored in it.
In addition, the higher the temperature, the more internal energy it have.
Boiling, Evaporation, Condensation, Melting and Solidification
i spent a long time digging for my last year's revision notes(again created by myself)!
Better appreciate my effort! :D
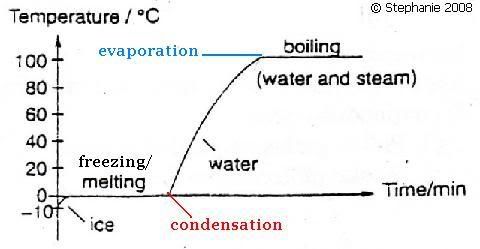
Below is the heating/cooling curve:
- Melting
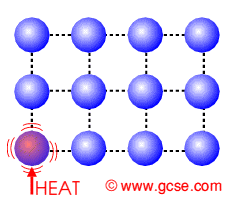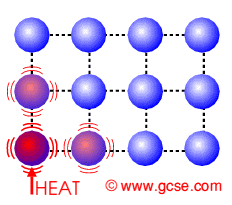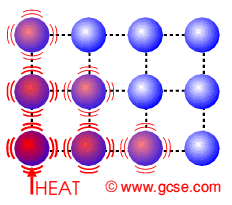
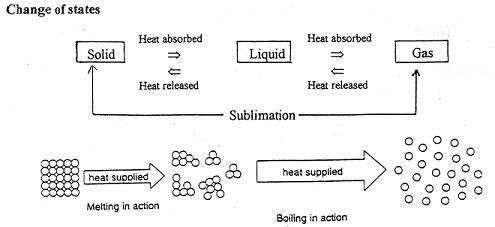
The molecules in a solid gain energy with the increase of surrounding's temperature to break strong intermolecular bonds. Melting occurs at a fixed temperature for every PURE substance, known as melting point. This involves a change of state, from solid to liquid.
Eg. Pure water melts at 0
° C.
- Solidification
It is the reverse process of melting and is the same meaning as freezing.
Solidification occurs at a fixed temperature for every PURE substance, known as freezing point. It involves a change of state, from liquid to solid.
Eg. Pure water freezes at 0°C.
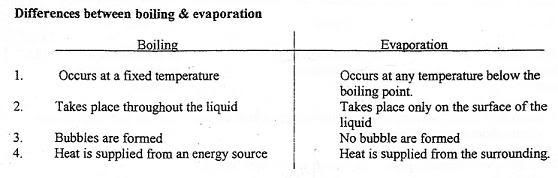
- Boiling
Molecules gain energy and break loose intermolecular bonds to move freely with the increase of temperature. Boiling occurs at a fixed temperature for every pure substance, known as boiling point.
Eg. Water becomes steam at 100°C.
Here's another Eureka! video on Evaporation & Condensation:
- Condensation
It is the reverse process of boiling. It involves a change of state.
Thermal energy is given out during condensation.
VIDEOTIME:
- Evaporation
Molecules gain energy from the surroundings to overcome intermolecular forces.
It occurs at any temperature, no definite temperature required.
Similar to boiling, it is the change of state from liquid to gas.
When evaporation occurs, thermal energy is taken away from object, resulting in cooling.
Eg. After a swim, your body feels cold due to evaporation of water.

Factors which affects rate of evaporation:
1. Temperature - High temperatures increase rate of evaporation.
2. Humidity - Rate of evaporation decrease with increasing humidity.
3. Surface Area - Rate of evaporation increase with more exposed surface area.
4. Movement Of Air - Rate of evaporation increase with greater movement of air.
5. Pressure - Reducing atmospheric pressure increases rate of evaporation.
6. Boiling Points - Liquids with lower boiling points evaporates faster.
Applications

Yes! That's the perfume i want! :P
Evaporation occurs when you experience a cooling effect after applying perfume.

Refrigerator makes use of the principles of evaporation and condensation to keep your food cool!

A candle melting. Change of state: Solid wax to Liquid wax

Your clothes can dry because water is evaporated from your wet clothes.
SUMMARY

Last but not least, i found a WATER CYCLE SONG!
|
An Introduction to Physics
Physics is a key discipline of Science.
It is the study of the natural world around us, and is divided into 5 main categories:
-General Physics
-Thermal Physics
-Light, Waves & Sound
-Electricity & Magnetism
Thermal Physics will be the general topic of this blog.
|
|
Unit 9 - Thermal Properties Of Matter
Objectives:
- Understand boiling, evaporation, condensation, melting and solidification.
- Understand what internal energy is.
- Applications of the different processes.
Internal Energy
Potential energy+Kinetic energy=Internal Energy!

Internal energy of a body is the energy stored in the body.
It is the sum of kinetic energy and potential energy of all particles in the body.
It increases with the temperature of the body and increases when the body changes state from solid to liquid and from liquid to gas.

The stretchable slinky have internal energy stored in it.
In addition, the higher the temperature, the more internal energy it have.
Boiling, Evaporation, Condensation, Melting and Solidification
i spent a long time digging for my last year's revision notes(again created by myself)!
Better appreciate my effort! :D
Below is the heating/cooling curve:


- Melting
The molecules in a solid gain energy with the increase of surrounding's temperature to break strong intermolecular bonds. Melting occurs at a fixed temperature for every PURE substance, known as melting point. This involves a change of state, from solid to liquid.
Eg. Pure water melts at 0
° C.
- Solidification
It is the reverse process of melting and is the same meaning as freezing.
Solidification occurs at a fixed temperature for every PURE substance, known as freezing point. It involves a change of state, from liquid to solid.
Eg. Pure water freezes at 0°C.
- Boiling
Molecules gain energy and break loose intermolecular bonds to move freely with the increase of temperature. Boiling occurs at a fixed temperature for every pure substance, known as boiling point.
Eg. Water becomes steam at 100°C.
Here's another Eureka! video on Evaporation & Condensation:
- Condensation
It is the reverse process of boiling. It involves a change of state.
Thermal energy is given out during condensation.
VIDEOTIME:
- Evaporation
Molecules gain energy from the surroundings to overcome intermolecular forces.
It occurs at any temperature, no definite temperature required.
Similar to boiling, it is the change of state from liquid to gas.
When evaporation occurs, thermal energy is taken away from object, resulting in cooling.
Eg. After a swim, your body feels cold due to evaporation of water.

Factors which affects rate of evaporation:
1. Temperature - High temperatures increase rate of evaporation.
2. Humidity - Rate of evaporation decrease with increasing humidity.
3. Surface Area - Rate of evaporation increase with more exposed surface area.
4. Movement Of Air - Rate of evaporation increase with greater movement of air.
5. Pressure - Reducing atmospheric pressure increases rate of evaporation.
6. Boiling Points - Liquids with lower boiling points evaporates faster.
Applications

Yes! That's the perfume i want! :P
Evaporation occurs when you experience a cooling effect after applying perfume.

Refrigerator makes use of the principles of evaporation and condensation to keep your food cool!

A candle melting. Change of state: Solid wax to Liquid wax

Your clothes can dry because water is evaporated from your wet clothes.
SUMMARY


Last but not least, i found a WATER CYCLE SONG!
|

An Introduction to Physics
Physics is a key discipline of Science.
It is the study of the natural world around us, and is divided into 5 main categories:
-General Physics
-Thermal Physics
-Light, Waves & Sound
-Electricity & Magnetism
Thermal Physics will be the general topic of this blog.
|
|